Corrosion is defined as the deterioration of materials through chemical or electrochemical attack. Corrosion requires the presence of both oxygen and water. Oxygen is available from the air but levels may be increased, especially in water by agitation. Water can be supplied by immersion, washing down, rainfall, dew and high relative humidity. Rainfall may not contribute to the corrosion process as it may wash away contaminants on the metal surfaces. Dew and high relative humidity however form a layer of moisture on the metal surface that can significantly influence the corrosion process.
On a macro scale, the rate of corrosion can be classified into categories, eg AS/NZS 2312 uses seven categories.
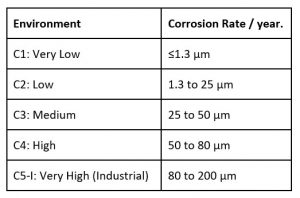
In addition to macro effects, local effects can significantly affect the rate of corrosion and convert a mild category into an aggressive corrosive environment. These can include:-
- Areas of poor housekeeping where there is a build-up of material that remains moist for long periods.
- Dripping taps, fire hydrants and wash down hoses. Prolonged periods of wetness can increase the corrosion rate by more than a factor of two.
- Areas that do not allow water to drain or are shaded from sunlight.
- Surfaces that are exposed to contaminants such as coastal salt but are protected from cleansing rain.
- The interior of large hollow sections which can experience very high relative humidity and temperatures due to the ingress of moisture through bolted connections and hatches and often are not inspected due to lack of access.
- Pollutants and other fallout such as industrial chemicals and solvents, fertilisers and other chemicals.
- Industrial processes such as coal wash-plants, fertiliser production, and other industries which produce corrosive materials.
- Dissimilar metals.
- Areas subject to wear and abrasion.
- Highly stressed components can corrode more rapidly.
Some of the direct consequences of steel corrosion include:-
- In steel sections that carry cyclic loads, the fatigue life decreases as the magnitude of variation in stress increases.
- A reduction in the thickness of section leading to a reduced load carrying capacity of the section and spreading to other parts of the asset.
- Failure of the corroded member which may cause distortion or failure of other members or may result in failure of a whole asset.
- Loss of contained material from structures such as bins and the like.
The options available for the remediation of corroded members include:
- Blast and paint either locally or extensively. Sections blasted should be inspected for the extent of loss of section prior to painting.
- Repair can include isolated repairs such as over plating or inlay plating.
- Replacement of the corroded section. This can include splicing a new section in or replacing the whole beam or column.
- Boxing-in or encapsulation. Boxing-in is useful for corrosion to webs of beam or for sections in shear but may not be beneficial for sections in bending where the flange is the critical component. Encapsulation in concrete may be useful for the base of columns for protecting the hold down bolts and keeping spillage and buildup away.
- Do nothing. This may be a viable option depending on the circumstances.
